There is currently a focus in the energy and transport sectors towards using hydrogen as fuel, mostly driven by the need to reduce pollution and CO2 emissions.
Background
Until large-scale hydrogen production from renewable energy sources becomes economical, the retrieval of hydrogen trapped in fossil fuels provides a way forward. Many ways exist to produce hydrogen from fossil fuels, with partial oxidation being one of the most competitive.
Most partial oxidation techniques use a catalyst which increases cost and introduces several other problems, for example catalysts are prone to poisoning and clog easily when fuel mixtures are too rich. Catalyst based systems also may suffer from long start-up times which makes them less attractive for on-board hydrogen production.
The Invention
Engineers at the Department of Engineering have invented a catalyst-free method of converting traditional organic fuels into hydrogen via a simple device which is compact, economical and easy to manufacture. This novel technology has applications in many industries: from automotive and transportation to power generation and chemical manufacture.
Typical applications include:
- Hydrogen generation for fuel cells (the most common types of fuel cell convert the chemicals hydrogen and oxygen into water, and in the process produce electricity)
- Hydrogen-enrichment of fossil fuels for motor vehicles
- Large-scale hydrogen production from natural gas.
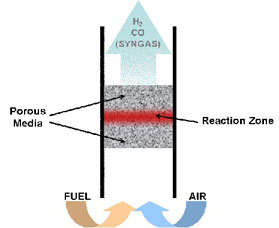
How It Works:
The hydrogen producing device transforms gaseous and pre-vaporised liquid fuels into mixtures of hydrogen and carbon monoxide without using a catalyst. It is based on the principle of "superadiabatic combustion", which allows very rich combustion (i.e. partial oxidation) zones to be stabilized. This is done by performing the combustion inside a porous inert solid. These porous solids can be either packed beds of spherical beads or porous foams. Foams, in particular, offer very quick start-up times. No catalyst is necessary and the device is robust.
"Clean" fuels like methane, methanol and iso-octane have been used, but also "dirtier" commercial-grade petrol has been successfully reformed into syngas (the hydrogen and carbon monoxide mixture from which the hydrogen is subsequently taken).
In summary the hydrogen producing device benefits are:
- Efficiency comparable to other partial oxidation techniques
- Economical to manufacture principally because it does not use a catalyst
- Quick start-up time
- Compact design suitable for on-board hydrogen production.
Status of Invention:
Patents have been filed on this invention and the University is now seeking to collaborate with industrial partners in order to commercialise the technology. The University will support the development of the technology through collaborative research projects and technology licensing.
Expertise can be offered in the use of this device in a wide variety of applications including:
- Syngas production
- Hydrogen production for fuel cells
- Hydrogen enrichment of traditional fuels
- Power generation
- Engine efficiency.
For further information, including a copy of the patent specification, or to discuss licensing proposals please contact:
Dr. Fauzia Farooq
Cambridge Enterprise
University of Cambridge
or the inventors
Dr. E. Mastorakos
Lecturer
Engineering Department
University of Cambridge
and
Mr. H. Pedersen-Mjaanes
PhD Student
Engineering Department
University of Cambridge


