
For her fourth year project, undergraduate Katy Cartlidge has evaluated whether a proposal known as the ‘ice volcano’ has the means to restore Arctic sea ice. Katy used theoretical modelling and experimental analysis to assess the feasibility and sensitivity of an ‘ice volcano’ to its environment, at a time when Arctic summer sea ice is declining at an alarming rate due to climate change.
A significant benefit of the ‘ice volcano’ is that it merely augments a natural activity and does not introduce any unfamiliar chemicals or processes into the environment.
Student Katy Cartlidge
The project titled Ice Thickening – Climate Repair investigated the use of the ‘ice volcano’ method to increase Arctic sea ice production in the winter. This method involves pumping seawater over a floating conical buoy, where it then freezes in the cold Arctic atmosphere to generate a growing cone of ice. A slotted pipe allows water to be pumped higher as the ice builds up. Once a height of three metres has been achieved, scientists say the newly-formed ice is likely to survive a summer melting season and become valuable ‘multi-year ice’, surviving at least two summers’ melt.1 This is a desirable outcome: thicker, stronger multi-year ice that is present in the summer, providing its benefits year-round.
Arctic sea ice has numerous benefits, such as: inhibiting methane release from permafrost melt; increasing the proportion of incoming solar radiation that is reflected; maintaining global currents that mediate weather patterns in the Northern hemisphere; and providing a habitat for polar wildlife. Scientists say that if current rates of ice decline are sustained, an ice-less summer will be seen in the Arctic within the next decade.
“A significant benefit of the ‘ice volcano’, in comparison to other proposed geoengineering projects, is that it merely augments a natural activity and does not introduce any unfamiliar chemicals or processes into the environment," said Katy.
Katy developed a simplified, two-dimensional model to predict the behaviour of an ‘ice volcano’ for fresh water at its freezing temperature and also fresh water at above its freezing temperature. This was then followed by experimental analysis, using a narrow channel that maps onto the two-dimensional model. The experiments were conducted in a walk-in freezer with a temperature of –18°C. Water, of varying temperatures and salinities (fresh water and water at the salinity of Arctic seawater), was pumped through the channel over a layer of cold ‘original ice’ and allowed to freeze or cause melting. The resulting change in ice thickness was then calculated.
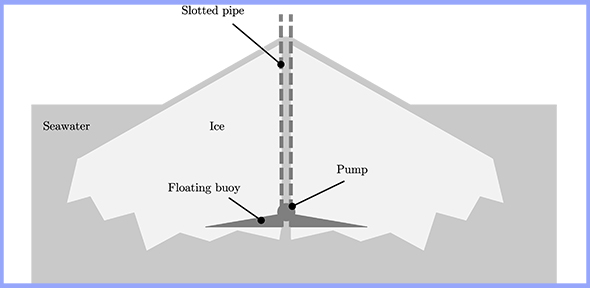
Diagram of an 'ice volcano'. Seawater is pumped from below onto a floating buoy, where it then freezes into a conical ‘volcano’. A slotted pipe allows water to be pumped higher as the ice builds up. The vertical scale is exaggerated. Credit: Katy Cartlidge.
Both theoretically and experimentally, the results were promising for water at its freezing temperature: ice formed readily and uniformly all along the channel, indicating an ‘ice volcano’ could be successful and uniform. Experimental ice build-up exceeded theoretical predictions for both fresh and salt water, potentially due to heat fluxes from the air that were neglected in the model.
However, results were less encouraging for water entering the channel above its freezing temperature. It was found that this results in a substantial loss of ice near the inlet, even for water just a few degrees above its freezing temperature, although ice is still created further down the channel. In the context of the ‘ice volcano’, this insinuates that the region of ice surrounding the pipe could be completely eroded within a few hours, creating an ‘ice doughnut’, unless the water is within a very narrow margin of its freezing temperature.
“This narrow range for acceptable water temperature poses operability issues,” said Katy. “At certain temperatures, an ‘ice volcano’ could even melt more ice than it creates, making it entirely unfeasible and counterproductive.”
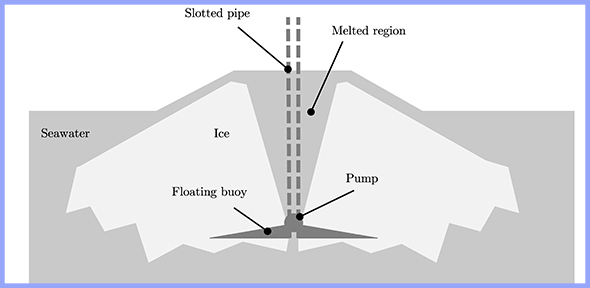
Presenting a situation where water enters above the melting temperature of ice. Ice is eroded around the pipe, forming a doughnut shape. Credit: Katy Cartlidge.
The experimental analysis was also extended to salt water, which had some notable differences, including a melting temperature higher than the freezing temperature of the salt water itself. The presence of salt alters the thermal properties of the water.
“This has a beneficial effect for the ice volcano, because if the ice melts at a warmer temperature than the water freezes, the water may be above its freezing temperature and still not melt the ice, increasing the range of operable temperatures,” said Katy. “However, if the salt water were warm enough, it would still cause the disastrous ice losses described above and render the ice volcano ineffective.”
Katy’s recommendations for further work on this topic include:
- The development of a theoretical model for a three-dimensional cone and also for salt water.
- Improving the accuracy of the model over long timescales, by accounting for convection, radiation and evaporation from the water to the atmosphere and removing the semi-infinite ice assumption.
- Investigating ripple and/or rivulet formation in detail and determining their impact on ice build-up.
- Continuing the experimental analysis with salt water, ideally using a cone and over longer timescales.
- Considering the magnitude and source of the power requirements for an ‘ice volcano’.
- Exploring feasible sea and air temperatures for ‘ice volcanoes’ and linking this to Arctic weather patterns to suggest how, where and when they might be implemented.
- Beginning detailed design for an ‘ice volcano’, and then building and testing a prototype in Arctic conditions.
Katy said: “When working in such a unique and fragile environment as the Arctic, it is necessary to take extreme care. Extensive trials in Arctic conditions, such as those that I carried out in the walk-in freezer, would be undertaken before contemplating any tests in the Arctic itself. The potential impact on ecosystems, climate and wildlife would be carefully evaluated at each stage, the formal approval process including ethics and stakeholder engagement for even small-scale field work would be exhaustive, and the experiments would be halted if unforeseen negative effects were observed.”
Katy is supervised by Dr Shaun Fitzgerald, Teaching Fellow in Engineering and Director of Research at the Centre for Climate Repair at Cambridge.
About the Centre for Climate Repair at Cambridge
The Centre for Climate Repair at Cambridge is working in affiliation with Cambridge Zero at the University of Cambridge to safeguard our planet from the disastrous effects of global warming.
The Centre’s mission is threefold: to reduce CO2 emissions; remove excess CO2 from the atmosphere; and refreeze the Arctic.
1 Peter Wadhams. Ice in the Ocean. Gordon and Breach Science Publishers, London, 2000.

