Kate Gross was just 36 years old when she died of cancer. Researchers at Cambridge – including her husband – are trying to ensure that others receive their diagnoses early enough to stop their cancer.
I think this programme couldn’t happen anywhere else, we have outstanding departments across all fields and an open-minded, collaborative atmosphere that grows research from the bottom up.
Dr Sarah Bohndiek, Department of Physics
It is October 2012. Kate Gross is a successful thirtysomething woman for whom everything seems to be perfect. Her job allows her to travel the world, rubbing shoulder with presidents and prime ministers. “My adorable twins are three, and their father Billy, is my soulmate, as well as the best-looking man I’ve ever kissed,” she wrote in her memoir, Late Fragments.
But Kate was about to learn that all was not, in fact, well. Returning from California, she was suddenly struck ill and ends up at Addenbrooke’s Hospital in Cambridge. “Inside me a lump of cells has broken free of the rules and spawned a tumour which has blocked my colon, crept through my lymph nodes and colonised my liver. Cancer is halfway to killing me, and I am completely oblivious to its presence.”
Following a CT scan and emergency surgery, Kate was eventually diagnosed with stage four colon cancer. “All this cancer talk is new to me,” she added, “but I do know there isn’t a stage five.”
In fact, the warning signs had been there for some time. In her typically frank way, she wrote: “I’ve always had a dodgy bottom. I presumed it was irritable bowel syndrome”. A sigmoidoscopy failed to spot the tumour, which was too far up the colon to see, but this only gave Kate and her doctors false confidence that everything was fine.
When the tumour was finally identified, the cancer had already metastasised and spread to her liver. According to her husband, Billy Boyle, only 5% of people diagnosed with colon cancer at such a late stage survive.
“Kate was not one of the lucky ones,” he says. His wife passed away on Christmas Day, 2014.
A breath test for cancer

Engineering alumnus Boyle, is determined that what happened to his wife should not happen to others: had his wife’s cancer been diagnosed at the earliest stage, she would have had a 95% chance of surviving, he says.
A decade earlier, Boyle had founded Owlstone, a spin-out from Cambridge’s Department of Engineering focused on detecting chemical agents. Using the same idea he had then, he has now spun out a second company, Owlstone Medical, to apply the technology for use as a ‘breathalyser’ for cancer. The breathalyser works by detecting specific chemicals in the breath, metabolites – waste products – produced by tumours.
“We know that cancer has an altered metabolism, even at a very early stage,” says Boyle, “so the goal is to try and pick up these markers when the cancer is most treatable. We want to give doctors better tools for making a differential diagnosis, so if someone comes to them with abdominal problems, for example, they are better at working out what it’s likely to be.”
Owlstone Medical has recently begun a clinical trial, in collaboration with researchers at the Cancer Research UK (CRUK) Cambridge Cancer Centre and Cambridge University Hospitals, to see whether this breathalyser might be used as a non-invasive test to identify a wide range of cancers –everything from bladder, breast, head and neck, kidney, oesophageal, pancreatic and prostate cancers to brain tumours – at a stage when more treatment options are available and the chances of surviving are much higher.
The pill on a string that could spot cancer
One of Boyle’s collaborators is Professor Rebecca Fitzgerald from the Medical Research Council Cancer Unit, co-lead of Cambridge’s Early Detection Programme, supported by CRUK.
“People are beginning to talk of cancer as being a ‘chronic disease’,” she says, “and that is the case for some cancers, where we can keep them at bay. But most cancers are diagnosed late, and once cancer has spread to the lymph nodes or into the blood, it’s going to be an uphill battle to treat it.”
As many as one in five cancers are identified only when they present as an emergency, when the cancer is more likely than not to be at an advanced stage. In fact, in more than three-quarters of these cases, the cancer is at stage three or four.
The reasons for this are multitude: patients are not good at spotting the warning signs or understanding their risk, there can be delays in get access to the appropriate diagnostic tests, and the tests themselves may not be reliable enough or cost-effective enough to roll out across the NHS.
Fitzgerald may be about to transform early detection of oesophageal cancer – cancer of the gullet. This is one of the cancers that has benefitted little from recent advances in treatment, in part because it is usually caught too late.
At present, patients with suspected oesophageal cancer have to undergo a gastroscopy – a camera down the gullet – but this is unpleasant and not good at distinguishing between the pre-cursor condition ‘Barrett’s oesophagus’, in which cells within the lining of the oesophagus begin to change shape, and malignant cells.
In fact, Barrett’s itself goes undiagnosed in more than eight out of ten cases; even though Barrett’s proceeds to cancer in only a minority of cases, this means that an opportunity to intervene before cancer develops is being missed.
She has developed the Cytosponge-TFF3 test, a ‘pill on a string’ that dissolves to reveal a sponge; when withdrawn, the sponge scrapes off some cells from the lining of the gullet which can then be examined using a simple antibody test for TFF3, a protein very specific to Barrett’s cells. It has been shown to work, and is now undergoing a UK-wide trial over 9,000 patients, also funded by CRUK, to compare its performance against the current endoscopy referral pathway. The trial is also examining how cost-effective and acceptable the test is for patients and whether practice nurses could administer the test in GP surgeries.
Overcoming language barriers
The Cytosponge and cancer breath test are just two of the projects supported by the Early Detection Programme, which spans early stage detection through to identification of cancer at a more advanced stage.
Fitzgerald’s co-lead in the programme is Dr Sarah Bohndiek from the Department of Physics, who is developing new imaging techniques for early detection and for monitoring progress of cancers. This collaboration between a clinical researcher and a physicist typifies the programme’s hugely interdisciplinary membership – around 250 researchers from 50 different departments in Cambridge: clinicians, basic scientists, physicists, engineers, social scientist, ethical and legal experts (in fact, it is probably simpler to say which departments are not involved).
“I think this programme couldn’t happen anywhere else,” says Bohndiek. “We have outstanding departments across all fields and an open-minded, collaborative atmosphere that grows research from the bottom up.”
Such diversity is not without its challenges. “We learned early on that there was something of a language barrier, so we had to do some work to help the physicists and engineers understand some of the clinical issues around early diagnosis and what makes it difficult in different cancers, for example,” says Fitzgerald.
To help address this problem, the team commissioned a series of infographics, which explained the scientific issues to the clinicians and the clinical issues to the scientists, in language that was familiar to each.
In fact, even bringing together researchers to develop these infographics proved fruitful – several pairings have received ‘pump-priming’ funding to take their projects forward. Pump-priming involves giving researchers small grants to help get a potentially risky project off the ground; if it proves promising, then hopefully a research funder or industrial partner will consider a larger grant to develop it further.
“We don’t expect that all of these projects will succeed, of course,” says Bohndiek. “If they do, then we’re not looking high risk enough.”
The nuclear physicist, the telecomms engineer and the holographic capsule
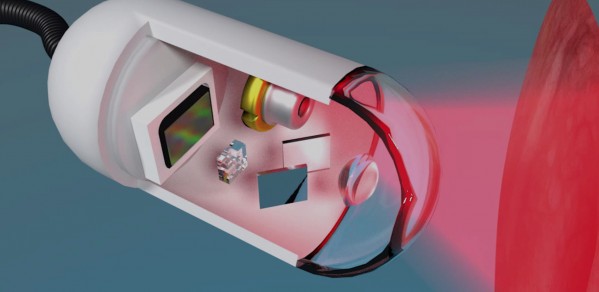
One such project runs in parallel with Fitzgerald’s own work on the early detection of oesophageal cancer: the development of a ‘holographic capsule’ that a patient would swallow and that could detect the presence of and help distinguish between ‘plasias’ – types of tissue growth. “I didn’t realise there were so many ‘plasias’ until I started this project," says Dr Catherine Fitzpatrick from the Centre of Molecular Materials for Photonics and Electronics. “There’s hyperplasia, dysplasia, metaplasia, neoplasia…”
Fitzpatrick, who began her research career as a nuclear physicist, including a short spell at CERN, had to quickly learn the unfamiliar terminology in her new field when she joined fellow engineer Dr George Gordon. He also came to medicine via an unusual route, having previously worked in telecommunications. (For both of them, the infographics proved to be a lifesaver.)
There are two challenges at the heart of Fitzpatrick and Gordon’s project: cost and contrast. Precancerous lesions in the oesophagus – dysplasia – are often missed because they are so difficult to identify using standard endoscopic imaging. With earlier detection leading to increased survival, the benefits of improving imaging contrast are clear. However, new techniques must also be low-cost in order to be widely accessible.
A number of contrast-enhancing techniques are in development; this project uses techniques from holography to generate contrast from small changes in the structure of the tissue itself. This means that no external dyes are needed, which simplifies the procedure. The tissue is illuminated with low-power laser light, and perturbations in the phase of the reflected light are analysed to identify suspicious lesions.
Fitzpatrick and Gordon plan to make this technique low-cost by implementing it in a miniature endoscope that sits inside a capsule the size of a large tablet, which the patient would swallow. In a sense, this is another ‘pill on a string’, only more hi-tech with a ‘string’ that could be a USB cable. The capsule would be withdrawn after it has done its work, and potentially even reused.
It sounds very futuristic, but Gordon recognises the need to make sure that it is both clinically-useful and cost-effective. “We want to avoid making something that has all the bells and whistles on it, but is too expensive and too complicated to use,” he says, and so they are working with Fitzgerald and other clinicians. “They don’t care whether a solution is simple or complicated, so long as it does the job.”
While it is too early to put a price on the capsules, Fitzpatrick and Gordon are working with low-cost components from the outset and expect them to be significantly cheaper than standard endoscopy.
Given that a 2015 report by Cancer Research UK estimated that more than 750,000 additional endoscopy procedures a year will be undertaken by 2020, a faster, cheaper endoscopic solution is much needed.
The economics - and ethics - of early detection programmes
Ultimately, the success of the programme will be if it leads to new ways of detecting cancer that are rolled out across the NHS. For this to happen, it will be essential to build health economics into their programme.
“Cancer treatment is incredibly expensive, especially with new targeted agents, chemotherapy, radiology, investigations, time in hospital, but if you can detect cancer sooner, the treatments get simpler,” says Fitzgerald. “But then of course, you’re testing more people, so the economics have to be worked out. You should be building this into your clinical trials.”
Fitzgerald expects that the economics “should stack up”, particularly if the tests are low tech and affordable and more thought is given to identifying and targeting those at greatest risk, either genetically or due to their lifestyles.
There are some in the medical community, however, who are suspicious of early detection and screening programmes. Open up the pages of the BMJ and you’re likely to read an opinion article or letter criticising their merits. One of reasons why, says Dr Stephen John from the Department of the History and Philosophy of Science, is concern about over-diagnosis.
Almost any screening programme is likely to pick up a number of ‘false positives’, people incorrectly diagnosed with cancer, who will then receive unnecessary medical treatment. “The problem is,” says John, “if you know that x% of all patients who receive a mastectomy have been misdiagnosed, you’ve still no way of knowing which x% of your patients that is. Similarly, you know that you’ve helped people, but you don’t know who you’ve helped.”
At the heart of this, he argues, lies the guiding principle of “First do no harm” (a phrase often erroneously attributed to the Hippocratic Oath). While screening will help those who do have cancer, it may lead to harm to those who unnecessarily undergo chemotherapy or radiotherapy as a result of misdiagnosis. As with medical interventions such as vaccination programmes and the widespread use of statins, overall the programmes do good, but not necessarily at an individual level.
“The response to this it that people have choice or consent, but a patient can only receive information in terms of their risk or probability, so is this really informed consent?” he adds.
John originally got involved in the Early Detection Programme after agreeing to give a talk to their researchers. Given his experience of how research projects often bring in the ethicists almost as an afterthought, he had expected to do his 30 minute talk and then go home, but was impressed by the interest and engagement from Fitzgerald, Bohndiek and colleagues. He is now working with them to look at the philosophical and ethical issues raised by early detection and screening programmes.
“Ultimately, a lot of the debates around over-diagnosis are philosophical debates, but these often feed into policy,” he says. “People are appealing to some moral principle. They might say ‘As doctors, our first duty is to do no harm, and look at what this test will do’. But then you can argue back that if that’s what they really think, then why agree with statins, why agree with vaccination?”
Cambridge is collaborating with the two other main centres, the Canary Center at Stanford University and the Oregon Health & Science University Knight Cancer Institute, and Fitzgerald and Bohndiek have plans to set up an Early Detection Institute that will form an integral part of an ambitious new cancer research hospital being mooted for the Cambridge Biomedical Campus. But given the potential to save so many lives, it is perhaps surprising that there are so few centres worldwide focused on early detection.
“I am writing this book to share the sum of a life,” wrote Kate Gross. “In a normal world, I would have been granted decades to say all of this. Fat, old and wearing purple, I would have bored my children and my children’s children with stories of the world I had known.”
This is the future that Boyle, Fitzgerald and Bohndiek hope to provide for others unfortunate enough to receive a cancer diagnosis.
Article by Craig Brierley
Late Fragments by Kate Gross is published by Harper Collins.

