A collaboration between the Department’s Nanoscience Centre and MedImmune is taking great strides towards safer and more effective treatment of type 2 diabetes and obesity.
Our years of research on how proteins and peptides can form nanostructures, has allowed us to take a potential medicine and redesign its delivery so as to make it far more effective.
Professor Sir Mark Welland
The Cambridge chemical engineers have been studying oxyntomodulin, a human peptide, which has the potential to be a safe and efficient form of treatment for both type 2 diabetes and obesity. One of the advantages of the new drug is that, unlike other treatments for type 2 diabetes, it will not cause the patient to gain weight – in fact, quite the opposite.
“There is evidence that oxyntomodulin both reduces appetite and causes a slight raising of body temperature and increase of the heart rate, which will help with weight loss,” says Sonja Kinna, a final-year PhD student, supervised by Professor Sir Mark Welland, who is investigating peptide self-assembly as a long-term drug formulation. “In addition to treating diabetes, we see it as a potential weapon to fight obesity.”
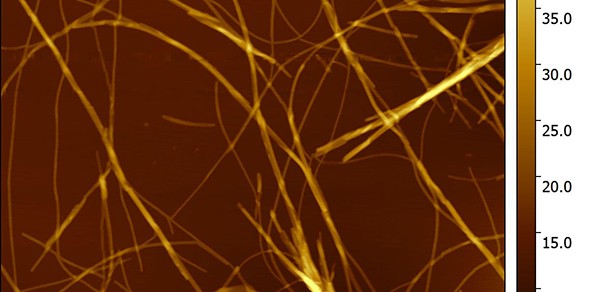
Sonja and the team have been examining the structural properties of the peptide, which can be stored in a fibrillar (or linear) structure. This structure is inert, but disassembles into a soluble state on being injected under the skin, triggering the release of insulin in the body.
The traditional treatment of type 2 diabetes involves injecting insulin directly into the patient. If too much insulin is applied, the patient can develop hypoglycaemia, but oxyntomodulin eliminates that risk by causing the patient’s body to produce its own insulin and balancing insulin production.
“We know peptides are a very safe and effective form of treatment,” says Sonja, “but the problem is that the body reacts to them as it would to proteins, treating them as food and therefore breaking them down. That’s why the ability to use oxyntomodulin’s fibrillated form is so important. We can use it as a depot from which the active peptide diffuses into the bloodstream over an extended period.”

Sonja at work in the lab. Credit: Sonja Kinna
Slow release from self-assembled structures creates a sustained action that circumvents the short half-life of peptides. This means the effect of the drug may last in humans for several days or even weeks. Although the drug is potentially effective in its free form, it would have to be administered frequently, perhaps as often as every four hours.
The team’s paper, 'Controlling the bioactivity of a peptide hormone in vivo by reversible self-assembly', won the Medimmune 2017 Global Excellence Award for the best publication of the year. The award recognises exceptional contributions to advance innovative science and deliver tremendous value to the MedImmune organisation.
“The best thing about this project has been the collaboration with MedImmune,” says Sonja. “It’s great because we [at Cambridge] study the structure of the peptides at a nanoscale, whereas the biologists from MedImmune look at the risk factors involved from an industry point of view. Together it works very well.”
The partnership is proving beneficial to both the University and MedImmune, and is potentially life-changing for millions.
“This work demonstrates how University research with a commercial partner can innovate medicine,” says Professor Sir Mark Welland. “Our years of research on how proteins and peptides can form nanostructures, has allowed us to take a potential medicine and redesign its delivery so as to make it far more effective.”
The Cambridge team uses atomic force microscopy to track signals and create images of the fibrils, which cannot be seen at all, even using the most powerful of optical microscopes. They also investigate the kinetics and thermodynamics of fibrillation and peptide release to better understand how they operate under various conditions.
There is, of course, much work to be done before any drug could appear on the market, and it must be carried out under very precise conditions. It is vital work, however. Not only does this study hold hope for better treatment for those suffering diabetes, but it also has implications for understanding diseases such as Parkinson’s Disease, which are caused when proteins fibrillate irreversibly.
“It’s very exciting,” says Sonja. “There is so much potential in this work, not only for medicine design and delivery, but also for understanding the development of diseases that are currently incurable.”
Words: Justine Bashford.

