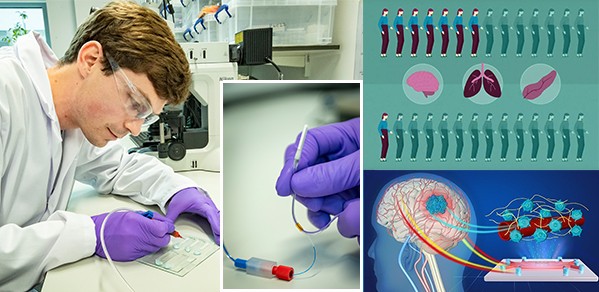
Researchers from the Department of Engineering are working collaboratively with scientists and clinicians on the development of targeted drug delivery technologies for three hard-to-treat cancers – mesothelioma, pancreatic cancer and glioblastoma (an aggressive form of brain tumour). These cancers are hard to treat because they come with their own defence mechanisms.
Having a specific clinical focus is necessary to best progress the technologies from the lab to the bedside in a timely fashion.
Professor George Malliaras
The researchers’ goal is to develop and validate novel drug delivery technologies to support the work of clinicians and improve survival rates for patients. The technologies can all be classed as platform technologies and are applicable to multiple cancers as well as other conditions.
The EPSRC Interdisciplinary Research Collaboration (IRC) in Targeted Delivery for Hard-to-Treat Cancers comprises five founding universities: Cambridge, Imperial College London, UCL, Glasgow and Birmingham, and more recently Nottingham, alongside several partner organisations. Survival rates for hard-to-treat cancers remain below 14%. Nine out of 10 people diagnosed with one of these specific tumours in the chest lining, pancreas and brain will succumb to the disease. This is why the IRC is developing high capacity nanoscale molecular vehicles and injectable gels, as well as implantable devices, to better target hard-to-treat cancers.
Nanoscale molecular vehicles with the aim of delivering drugs exactly where they are needed and nowhere else. They are adaptable and offer high drug carrying capacity and controlled release.
Gels that can be injected onto the cavity surface of the brain after a tumour has been removed, gradually release anti-cancer drugs to eliminate any residual tumour cells. Combining gels and molecular vehicles enables opportunities to enhance therapies and control delivery timescales.
Implantable devices that deliver high concentrations of drugs directly to tumours that are difficult to operate on.
The IRC has created the animation above to help explain the goals of their technologies.
“Having a specific clinical focus is necessary to best progress the technologies from the lab to the bedside in a timely fashion,” said Professor George Malliaras, Director of the IRC and Prince Philip Professor of Technology. “We develop materials and devices and explore their structure and properties before transitioning to models and validating processes, always driving development towards human trials.
“To this end, we engage with clinicians from the very start to map the real-life needs for technology development, what has to happen when, where the path leads, and what and where the bottlenecks and obstacles might be. A truly interdisciplinary process, our scientists, engineers and clinicians are all adept at communicating across traditional discipline boundaries.”
Dr Ronan Daly, Associate Professor in the Science and Technology of Manufacturing, and his team (Research Associates Dr Niamh Fox, Dr Etienne Rognin and Dr Qingxin Zhang), from the Institute for Manufacturing (IfM), part of the Department of Engineering, are supporting all of the drug delivery technology areas through the development of simulation tools. One of these enables IRC researchers to accurately explore how a drug would diffuse in the brain and carry out experiments with great precision to consider different modes of operation for an implantable deliverable device.
Dr Daly said: “As new platform technologies are created to solve the world's biggest challenges, we also need new infratechnologies to support their development. These are the underpinning tools that help with translation. They are purpose-built to enable rapid understanding and feedback to the researchers to help with design iterations. We support the technology leaders by developing these tools so they can better predict how best to help a patient.”
Dr Shery Huang, Associate Professor in Bioengineering, has been working with colleagues from the Department of Clinical Neuroscience and the Department of Paediatrics, on the development of a microfluidic device to perform comparative studies to understand the behaviour of patient-derived glioblastoma stem-like cells and their interactions with the blood-brain barrier.
“The novel creation of the flowable brain microvessel, the ability to perform gene expression analysis, and the use of live-cell imaging and image analysis aided our understanding of brain tumour progression,” said Dr Huang. “The customisable nature of the device, opens up the possibility for clinicians to be able to study tumour susceptibility to new treatments.”

