
Department receives one of the two 2024 BHF Translational Awards from the British Heart Foundation.
The current standard of care involves synthetic non-degradable grafts which suffer from high levels of complications and lack a regenerative ability. Our aim is to lessen the impact of dialysis on patients’ lives with our next generation, off-the-shelf biological grafts.
Professor Athina Markaki
The BHF Translational Award will fund a project seeking to replace synthetic haemodialysis grafts, used to connect an artery and a vein to facilitate kidney dialysis, with a patented biological graft and demonstrate lower risks of infection, thrombosis, and other complications, thereby leading to reductions in surgical interventions and reduced demand for hospital access.
The 3-year project has received £741K in funding and is led by Professor Athina Markaki at the Department of Engineering.
Approximately 2.5 million people worldwide currently receive dialysis treatment, and this number is expected to reach 3.4 million by 2030. While a surgical connection made between an artery and a vein, known as fistula, is considered the gold standard, synthetic arteriovenous access grafts e.g. expanded polytetrafluoroethylene (ePTFE) are used when the patient is ineligible for a fistula (blocked, damaged or thin veins), or has a previously failed fistula. However, they are prone to infections (11-15% annual hospitalisation rate), and occlusion (~50% of all ePTFE grafts are abandoned after 18 months), requiring regular maintenance and intervention.
To enable future regulatory review and human clinical trials, medical devices such as a graft, require validation in small animal and large animal models as a proof-of-concept. With support from Cambridge Enterprise, Cambridge Academy of Therapeutic Sciences (CATS), the School of Technology’s Seed Fund, the Office for Translational Research (OTR) and Engineering for Clinical grants (ECP), we have de-risked the project using ex vivo testing and small animal studies.
The BHF Translational award will provide pilot data in a large animal model where clinically-comparable surgeries can be performed. Following validation with vascular access grafts, the team intend to pursue more invasive grafts for other cardiovascular applications (e.g. aortic aneurism, coronary artery bypass procedures). The work will be carried out in collaboration with different departments and institutes across the University of Cambridge (Surgery, Medicine, Queen’s Veterinary School, and Cambridge Stem Cell Institute), and Addenbrooke’s and Royal Papworth Hospitals. The team’s co-investigators are Professor Kourosh Saeb-Parsy, Professor Sanjay Sinha, Professor Gavin Pettigrew, Professor Marie Aude Genain, and Dr Martin Goddard and Researcher co-lead is Dr Alexander Justin.
Professor Markaki says: “The current standard of care involves synthetic non-degradable grafts which suffer from high levels of complications and lack a regenerative ability. Our aim is to lessen the impact of dialysis on patients’ lives with our next generation, off-the-shelf biological grafts. We are grateful to Cambridge Enterprise, Cambridge Academy of Therapeutic Sciences, the School of Technology, the Office for Translational Research and the Department of Engineering for their support over the past few years which enabled us to obtain proof of concept data necessary for the procurement of external translational funding from BHF”.
Dr Justin adds: “The internal support was instrumental in advancing the project to a stage competitive for an external translational award. Additionally, the Impulse Programme and the Chris Abell Postdoc Business Plan Competition were key in developing a strong case for commercialisation.”
BHF Translational awards support the development of technologies with transformative potential to human cardiovascular health from the proof-of-concept stage to being ready for the commercial market. Proposals are expected to demonstrate a strong intellectual property position and a development proposition that could attract follow-on investment.
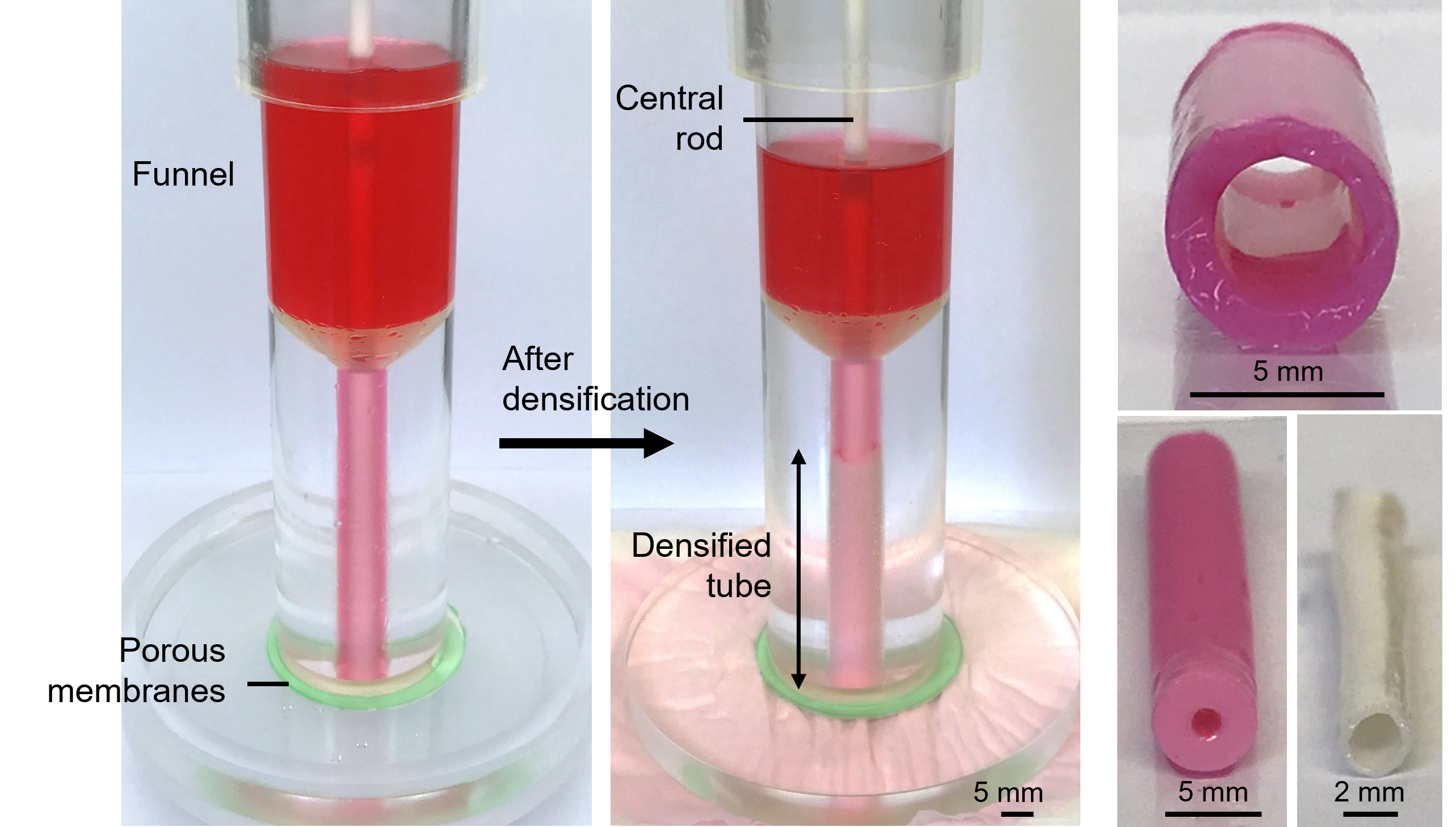
The images above show the patented method for de novo production of biological grafts from “densified” collagen hydrogel, through an approach which does not require the use of cells or decellularization methods, supporting true off the shelf capability.

